The unique chemical and physical properties of metals mean that they are extensively utilised by industry in a huge variety of applications, including electronics, transport, materials, industrial catalysts and chemicals. The increased consumer demand from a growing population worldwide with rising aspirations for a better life has resulted in concerns over the security of supply and accessibility of many of these valuable elements. The long term security of elemental supply has become an important issue at local (industrial), national and regional levels with the EU being one of the most badly affected due to its relatively small known mineral reserves. The increasing scarcity of many elements is most dramatically illustrated using the instantly recognizable form of Mendeleev’s Periodic Table modified to show what is sometimes referred to as “Elemental un-sustainability” (figure 1).
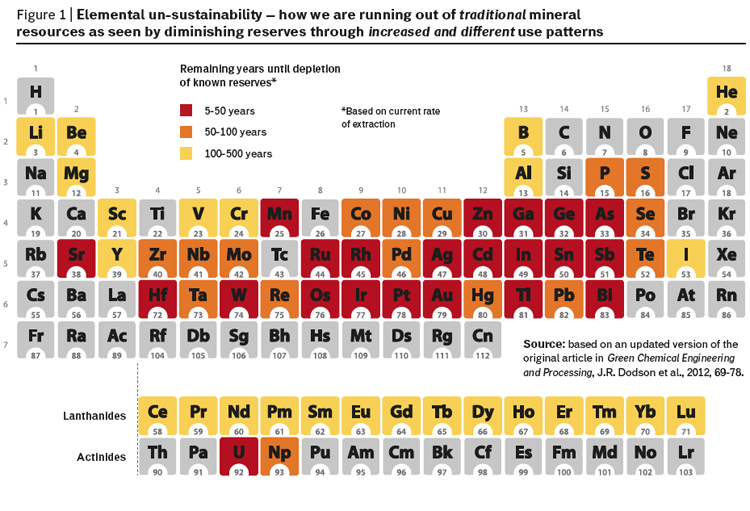
Elements are not “running out” or being destroyed, but rather are being dispersed throughout the technosphere, making recapture both highly problematic and often prohibitively expensive. These challenges must be tackled through the development of multidisciplinary partnerships, which adopt sustainable, holistic approaches consistent with recovery and reuse. Within this framework it is also important to consider the triple bottom line of sustainability, i.e. the environmental, societal and economic effects of these elements and their use.
Many elements currently have low end-of-life recycling rates, and the overall efficiency of the recycling process is predominately dictated by the collection of waste metal directly after use. Both platinum and palladium already have well established recycling routes, as their use is dominated by the automobile catalyst industry. Their recovery after use is well understood, and collection is inherent in the current processes for dealing with end-of-life catalytic convertors. This is in contrast to the vast majority of elements which are much more difficult to recycle due to their low concentrations and dispersion in a wide range of waste streams. We make the problem greater by more wealthy regions exporting large volumes of metal-rich wastes to less wealthy countries where the limited availability of modern technologies and poor health and safety standards add to the environmental impact of the waste streams. By considering the social, economic and environmental impact of an element’s life cycle, it is possible to highlight opportunities for the implementation of green and novel technologies for the recovery of elements. Increased rates of recycling will help us move towards a circular economy and reduce reliance on element extraction and purification hopefully without having to impose a degree of dematerialization (reduce use of resource). A dematerialization policy might take the form of “critical element quotas” but society might be highly resistant towards this and it could be very difficult to implement (especially where living standards and personal expectation are rapidly rising). The different stages in a metal lifecycle are illustrated in figure 2.
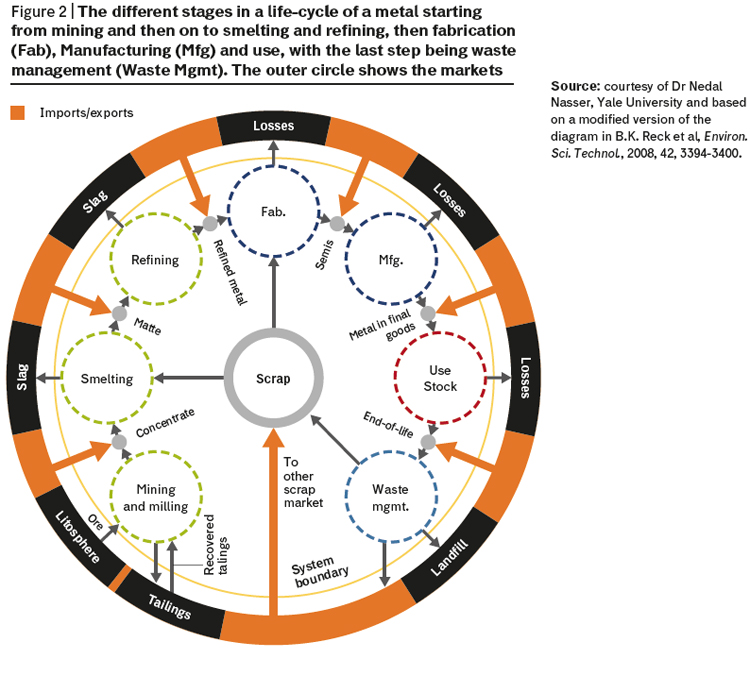
For example, it is estimated that some 40 metric tonnes of platinum are lost each year during the mining stages with another 20 MT being lost during the downstream processing and use of the element. Despite the relatively high level of recycling and high value of platinum, we still lose over 50 MT per annum in waste management.
Too Complex to be Circular?
A typical modern mobile phone contains over 40 different elements including arsenic, copper, gallium, gold, indium, magnesium, palladium, platinum, silver, tin and tungsten, all of which have been listed on the high current supply risk index by the British Geological Survey. They also have in common the worrying fact that the major suppliers and holders of reserves are outside the EU. Each generation of new phones seems to have an increased number of elements contained within them. Each year is also seeing an increase in the number of elements we use that we also consider to be at risk. We are making new ore discoveries but the rate of these new discoveries is going down and the average cost of extracting the elements is going up. According to the US Geological survey, the number of annual new ore discoveries halved between 2005 and 2010 yet we spent twice as much in 2010 on exploration. Like with petroleum we have used up most of the easy reserves and whats left is going to be in increasingly difficult places which we will only be able to exploit at high economic and environmental cost.
The increasing complexity of modern articles is not only increasing the use of more and more elements, it is making their recovery more difficult. Ironically, the natural complexity of mineral ores that contributes to the low efficiency and high environmental impact of metal extraction and purification is being repeated in our man made items. Ores that have taken millions to year to migrate close to the earth’s surface are highly complex; for platinum for example, the concentrations in mined ores is typically a few grams per metric tonne of rock (i.e. a few ppm) and it has to be separated from a plethora of other metals that can include copper, nickel, rhodium, iridium, ruthenium, tin, lead, arsenic and others. Having put so much effort into isolating the metals, it seems perverse that we then put so many of these into complex multi-element articles. This circle of complexity is reminiscent to what we do with our synthetic organic articles such as personal care products whereby we have effectively waited for nature to partially reduce the natural molecular complexity of biomass into fossil resources (essentially converting carbohydrates into hydrocarbons some of which have migrated towards the earths crust from where we extract them); our chemical industry than adds molecular complexity to these simple molecules so as to create the effects we desire, and then our process industries mix the resulting chemical compounds to create consumer products. In all cases, this man-made complexity makes any recycling of the resources in mineral and organic articles much more difficult.
Not a Lot of Recovery
Most of what we produce ends up in waste. The economic miracle of the 20th century that brought some one billion people into a comfortable lifestyle with high resource consumption was fed with non-renewable resources and based on a linear economic model of mine-process-consume-dispose, and most of the disposal was into landfill sites (figure 3).
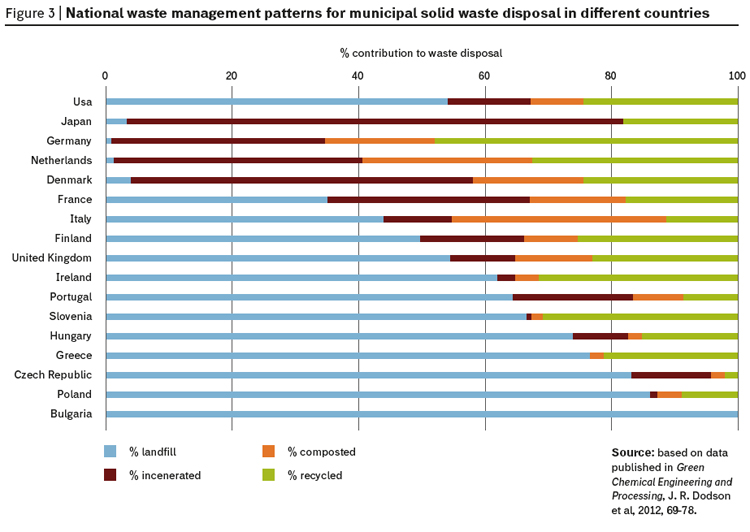
Given the data in this article and information on the way we have treated resources, its hardly suprising to see that during this period of economic and consumption growth, we have not recycled many of our precious elements (figure 4).
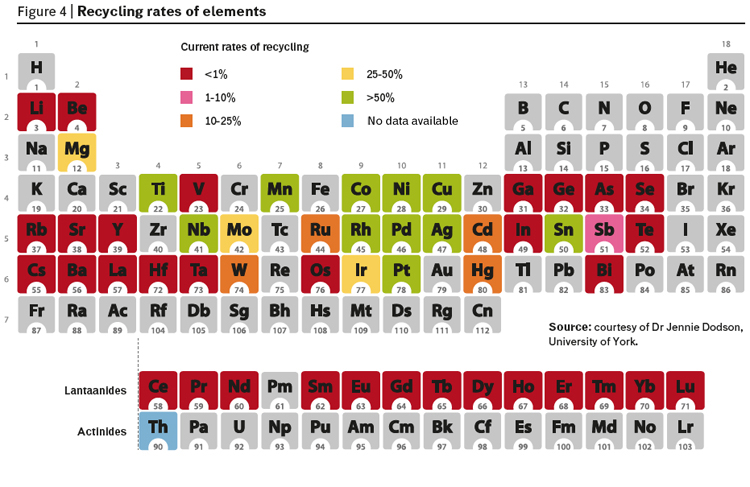
If we are running out of critical elements that are easily accessible and we are not recovering what we use then is clear that the growth in consumption resulting from an even larger number of people from developing countries cannot be supported by the same consumption model. But can we replace it with another linear model based on different earth-abundant elements? There has certainly been an increase in the research on alternatives. In the world of organic articles this is being driven by a combination of scarcity (diminishing traditional petroleum resources are used to manufacture over 90% of the articles) and legislation restricting the use of many compounds (due to increasing concerns over the toxicity and environmental impact of chemicals in common use including solvents, agrochemicals, flame retardants, surface coatings and numerous additives such as phthalates). For minerals the drivers are mostly scarcity driven and with the added concerns from regions with low natural virgin mineral resources. In both cases though for rather different reasons, the EU is taking the lead and it will be interesting to see if the EU completes the analogy by introducing legislation to restrict the use of what it now recognizes as critical elements. We should critically analyse the probability of success in finding alternatives for critical metals of which the platinum group metals are often considered to be the prime example.
Platinum Group Metals
Lets look at arguably the most famous group of metals, the platinum group metals (platinum, palladium, rhodium, ruthenium, iridium and osmium) or PGMs. PGMs possess an incredible array of physical and chemical properties that make them uniquely suited for a multitude of applications including automotive (including catalytic converters), catalysts in chemical and pharmaceutical production (many modern drugs are manufactured using process steps that use palladium in particular), petroleum refining, electronics (including hard disk drives), medical and dental (including anti-cancer drugs such as cisplatin) and ofcourse in jewellery. The value of these metals and the diversity of their applications has inevitably encouraged the consideration of (typically cheaper) alternatives but generally the alternatives that have been identified are themselves often, but not always other PGMs. For example for platinum the following alternatives have been proposed:
- Autocatalysis / Palladium;
- Electronic / Alloys of palladium;
- Medical / Some chromium alloys for some applications;
- Chemical catalysis / Other PGMs;
- Petroleum refining / Molybdenum.
The relatively common suitability of other PGM alternatives is hardly suprising given the similarity in properties of this closely related group of metals. Where non-PGM alternatives have been proposed there are generally severe limitations including poor performance or the need for major redesign of associated equipment, for example expensive chemical plant modifications when the alternative is not a drop-in replacement. In some cases there is no known adequate replacement; for example, in the enormous application area of automobile catalysis: despite thousands of other possibilities being tested, none can come close to the performance(s) of the PGMs. This application has a daunting set of requirements in terms of the chemical reactions the catalyst must promote (including the oxidation of carbon monoxide and unburned hydrocarbons) and the poisons such as sulphur, that it needs to tolerate.
This does not mean that the search for alternatives to critical metals and other important elements that are not readily abundant is in vain. In the world of chemical and pharmaceutical process catalysis for example, there have been some exciting research developments on using earth abundant metals rather than PGMs and other scarce metals as catalysts. In the same field, we have seen the growth of what are referred to as organo-catalysts that are completely metal-free. Of course, as with the growing concerns over some organic articles like solvents, we must not “throw the baby out with the bathwater”. We can and should expect to continue to use at least some metals and at least some solvents – we need them for too many important applications where in many cases there are not adequate replacements. But there are certainly many applications that are not so important and many cases where alternatives can be used with little added economic hardship or application value reduction. The number of successes is testament to this and once again proves the adage that necessity is the motherhood of invention.



